

The molar mass of an atom is directly read in the periodic table. The Avogadro constant N A 6.02214076×10 23 mol-1 is the number of particles that are contained in one mole of a substance. This number corresponds to the number of Avogadro `N_A`.
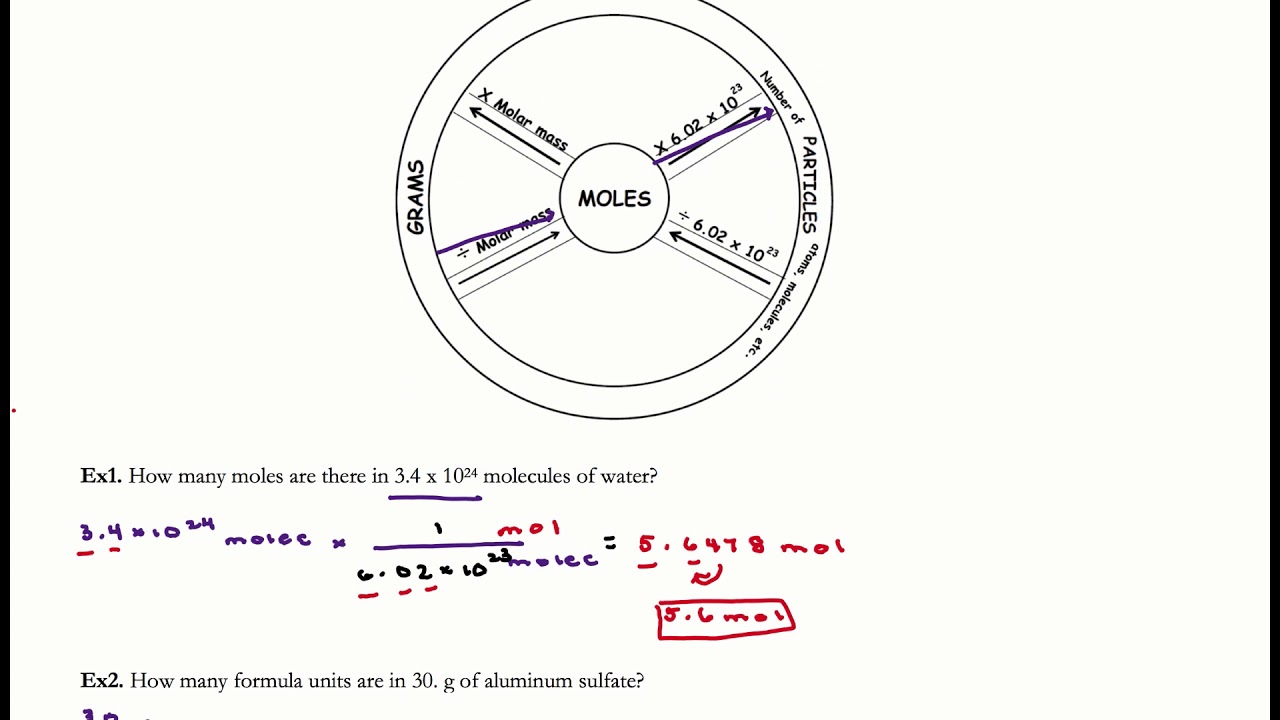
The symbol used is M and the usual unit is g/mol (corresponds to kg/mol in the international system).Īs a reminder, a mole of a chemical element is the amount of substance containing `6,02214076*10^23` of elementary entities of that element (atoms, molecules, elemental particle, etc.). The molar mass of an atomic or molecular chemical element is the mass of a mole of that substance. If you type SI for Silicon instead of Si, then the calculator will interpret this formula as. Examples: Fe, Co and Xe not FE, CO, XE, fe, ce and xe. The symbol of an atom with two letters has the first letter in uppercase and the second in lowercase. The symbol of an atom with a single letter is always written in capital letters. So, you need to choose "disable" field 1 to calculate field 2 and disable field 1 and 2 to calculate field 3.ĭo respect the uppercases and lowercases letters when you enter a chemical formula in the calculator above (3rd field). Type in your own numbers in the form to convert the units Quick conversion chart of mole to molecule 1 mole to molecule 6.0221415E+23 molecule 2 mole to molecule 1.2044283E+24 molecule 3 mole to molecule 1.80664245E+24 molecule 4 mole to molecule 2. Learn vocabulary, terms, and more with flashcards, games.
Moles to particles calculator how to#
3rd field: the molar mass of the formula entered (atom or molecule). Use this page to learn how to convert between moles and molecules. Start studying Chemistry A - Calculations (mole to grams & particles to mole conversions). 2nd field: the molecule mass of the selected molecule (choice from a list of common molecules) 1st field: the molar mass of the selected atom (choice from the 118 elements of the periodic table). The tool calculates in order of priority:


 0 kommentar(er)
0 kommentar(er)
